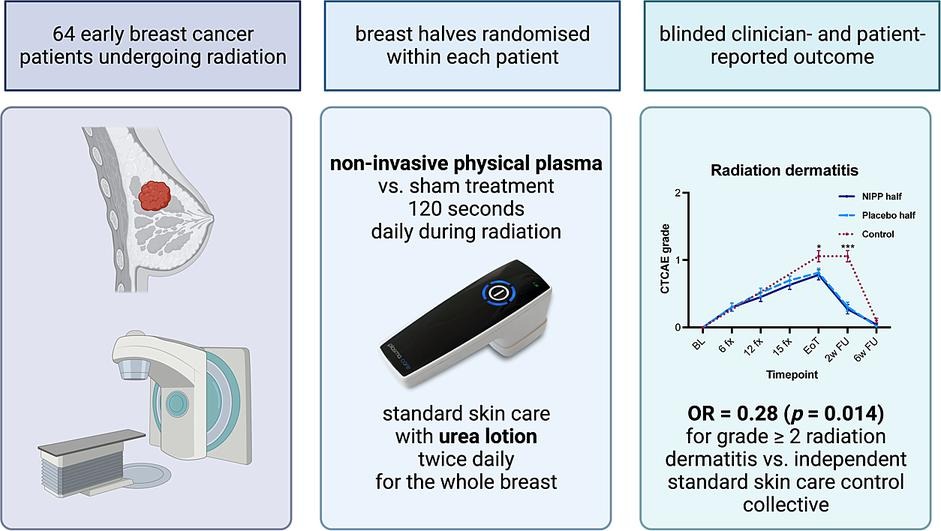
Objective:
To investigate the effect of topical non-invasive physical plasma (NIPP), […] on the prevention of acute radiation dermatitis (RD) during and after whole breast irradiation (WBI). For this purpose, the lateral and medial halves of each patient’s breast were randomised to daily treatment with either 120 s NIPP or placebo. Acute skin toxicity was assessed weekly in a blinded fashion for each half of the breast separately.
Results:
64 patients were included. There were no significant differences between the breast halves. The tolerability of NIPP was excellent and no side effects were observed.
Discussion:
Although there were no differences between the intra-patient randomised breast halves, the overall incidence and severity of acute radiation-induced skin toxicity was lower compared to a prospectively collected SoC cohort. Our data indicate the potential benefit of NIPP in RD prevention. A randomised trial with a physical control group is warranted to confirm these promising results (DRKS00026225).
Study design:
Monocentric phase 2 study, March 2022 to September 2023.
Patients and criteria:
Inclusion criteria:
Age ≥ 18 years, breast-conserving surgery and a moderately hypofractionated radiotherapy radiotherapy regimen. A normofractionated sequential boost to the tumour bed was allowed.
Exclusion criteria:
Synchronous metastatic disease, mastectomy, reconstruction with breast implant, alternative fractionation regimens, history of irradiation to the ipsilateral breast, any pre-existing dermatological skin disease, active dermatitis, current treatment with topical or oral corticosteroids, and patient refusal to participate in the study.
Read the full article: https://www.ctro.science/article/S2405-6308(23)00124-6/fulltext
Author Contributions: Cas Stefaan Dejonckheere, Julian Philipp Layer, Younèss Nour, Katharina Layer, Andrea Glasmacher, Shari Wiegreffe, Arne Fuhrmann, Lara Caglayan, Franziska Grau, Gustavo Renato Sarria, Davide Scafa, David Koch, Martina Heimann, Christina Leitzen, Mümtaz Ali Köksal, Fred Röhner, Thomas Müdder, Egon Dejonckheere, Frederic Carsten Schmeel, Teresa Anzböck, Kira Lindner, Anne Bachmann, Alina Abramian, Christina Kaiser, Andree Faridi, Alexander Mustea, Frank Anton Giordano, Matthias Bernhard Stope, Leonard Christopher Schmeel